by
Robert Ames
THE PROTEIN in our muscles undergoes a continual
process of synthesis and degradation. Athletes and weightlifters know
that after a strenuous workout, muscle tissue is damaged and needs time
to regenerate and be repaired. If we provide sufficient rest and
adequate nutrition, the body will usually overcompensate and produce
stronger and larger muscles.
Most analyses of strength training are concerned with two phases: the
work phase where we apply physical stress to our muscles to cause
microtrauma and resulting overcompensation, and the anabolic phase in
which we seek to enhance protein synthesis. But there's a third phase, a
period of breakdown and recovery, which is rarely discussed. We are told
to rest and to do what we can to avoid cortisol, but very seldom is
there any mention of the signal molecules which accomplish the work of
bringing the body back to a state of homeostasis.
Weightlifters talk of "destroying" their legs in a squat workout; of
loading the muscles with weight and stressing them until they are barely
able to function. What are the mechanisms that permit the body to
recover from such punishment? Is it possible to optimize recovery so
that less tissue is broken down, and we get into the anabolic phase more
quickly?
The essay that follows will deal with some very technical concepts.
I'm including a glossary containing brief definitions for the scientific
terms used. The purpose of such a technical essay is twofold: First, it
introduces some basic ideas in cell biology that will enable a better
understanding of exercise physiology. Once the basic concepts are
learned, one can view this area of science not as a collection of
disparate facts, but as a coherent system that runs on a logical -- but
complicated -- basis. Secondly, by going into detail concerning the
stages whereby the body detects damage, disposes of damaged tissue, and
ultimately replaces or strengthens the affected tissue, we can identify
areas where we can intervene with nutrition or chemical agents to reduce
damage and enhance our muscular gains. Also, it may be possible to take
advantage of this intimate knowledge to design training protocols that
coincide with the catabolic and anabolic stages that follow exercise.
Cytokines and Interleukins
There are four types of signaling molecules in the body:
neurotransmitters, endocrine hormones, autacoids and cytokines.
Cytokines are soluble proteins which act non-enzymatically to regulate
cell function. There are various types of cytokines, among them being
interleukins, hematopoietic regulators, interferons, growth and
differentiation factors and chemotactic polypeptides. Interleukins
(abbreviated IL) are cytokines that are produced by leukocytes (white
blood cells) and that function during inflammatory responses. They may
also be produced by other types of cells. Typically, interleukins have
the twin properties of pleiotropy and redundancy. Pleiotropy means that
an interleukin may have several different effects, depending on the
environment and the tissue acted upon. Redundancy in this case refers to
the ability of other cytokines (interleukins or not) to produce some of
the same effects as the interleukin being studied. This redundancy can
be due to the fact that receptors for interleukins often share common
subunits, or it may also be caused by identical effects on transcription
factors or on the DNA itself.
As of October 1998, eighteen different ILs have been described. We'll
be focusing on interleukin-6 (IL-6), which has some special properties
that make it interesting to bodybuilders. For those who might be
curious, here is a brief survey of all the interleukins:
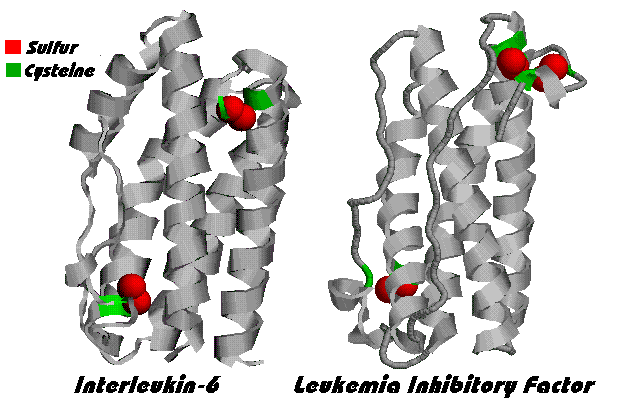
Figure 1. IL-6 molecule
Interleukin Description
IL-1 An inflammatory cytokine. One of the first cytokines to be
secreted following trauma, infection, etc. Induces IL-6.
IL-2 Secreted by Type 1 T-helper cells (Th1) of the immune
system. Stimulates cell-mediated (as opposed to antibody-mediated)
immunity. Generally a beneficial cytokine. IL-2 levels decline with age,
but are upregulated by DHEA.
IL-3 Growth factor for hematopoietic cells. Acts in a similar
fashion as granulocyte-macrophage colony-stimulating factor (GM-CSF).
Secreted by activated T lymphocytes, it induces formation of
macrophages, neutrophils, etc. Also induces secretion of immunoglobulin
from B cells.
IL-4 Anti-inflammatory cytokine. Related to IL-13. Released by
activated T cells, it initiates the humoral response (antibodies).
IL-5 A B-cell growth and differentiation factor; also stimulates
eosinophil precursor proliferation and differentiation. Secreted by
activated T cells.
IL-6 Pro- (and sometimes anti-) inflammatory cytokine.
Pleiotropic. The subject of this article. Main signal of cellular
injury, and main mediator of the body's response to injury. Most
important stimulator of acute phase proteins. Has an important role in
hematopoiesis. Produced by a variety of cells.
IL-7 Growth factor produced by a number of different cells.
Unlike other interleukins, IL-7 in not redundant, i.e. its function can
not be duplicated by other cytokines. It is required for lymphocyte
development.
IL-8 Pro-inflammatory. A chemokine. Can be induced by IL-1 and
lipopolysaccharide from bacteria. Produced by many different cells.
IL-9 Cytokine produced by T cells, particularly when mitogen
stimulated, that stimulates the proliferation of erythroid precursor
cells. May act synergistically with erythropoietin. Synergizes with IL-4
to produce immunoglobulins.
IL-10 Anti-inflammatory. Produced by Th2 cells, plus some B cells
and monocytes. Stimulates growth of stem cells and thymocytes.
Stimulates B and T cell development. Suppresses cytokine production by
macrophages.
IL-11 Pleiotropic cytokine originally isolated from bone marrow.
Stimulates B cell maturation, and production of erythrocytes (red blood
cells) and megakaryocytes. Synergizes with IL-3. Induces synthesis of
acute-phase proteins in the liver.
IL-12 Formerly known as Natural Killer Cell Stimulatory Factor (NKSF).
Produced by monocytes, macrophages, B cells, NK cells. Acts
synergistically with IL-2 to transform T cells into cytotoxic T
lymphocytes (CTLs). Stimulates the proliferation of activated T cells
and NK cells and induces them to produce interferon-gamma.
IL-13 Anti-inflammatory. Related to IL-4. Produced by activated
Th2 cells. Inhibits IL-6. Stimulates antibody production.
IL-14 A high molecular weight B lymphocyte growth factor. One of
the least researched cytokines.
IL-15 Anabolic for skeletal muscle. IL-15 receptor contains some
sub-units with the IL-2 receptor.
IL-16 Pro-inflammatory. Formerly called Lymphocyte
Chemoattractant Factor.
IL-17 Pro-inflammatory. Produced by T cells. Activates NF-kappaB.
IL-18 Pro-inflammatory. Induces the cytokine interferon-gamma.
Interleukins
and the Acute Phase Reaction

Fig. 2. Top mouse was continuously exposed to IL-6.
Bottom mouse received antibodies against IL-6. From
DeBenedetti1997.
EXERCISE modulates the immune system.
Following even moderate exercise, there is an elevation in the
number of neutrophils, the most common type of white blood cell
(Boyum1996, Cannon1994, Tidball1995).
After acute or short-term exercise, the total number of
lymphocytes increases, but if the exercise is intense and of long
duration the number of lymphocytes decreases (Pedersen1997). A lack
of glutamine resulting from exercise stress can impair the ability
of lymphocytes to proliferate and to function (Sharp1992,
Rohde1998).
Prolonged low intensity exercise may lower levels of
interleukin-6 in the blood (Boyum1996), while intense or eccentric
(negative) exercise causing muscle damage induces a dramatic rise in
this cytokine (Bruunsgaard1997, Weinstock1997, Ullum1994).
In short, intense exercise increases cytokines which may act to
break down muscle, while extensive exercise decreases cell- mediated
immunity (i.e. the ability of Natural Killer cells, cytotoxic T
lymphocytes, and phagocytes to eliminate potentially harmful cells
and materials).
Massage therapy has been shown to increase cell-mediated immunity
(Ironson1996), so there may be some benefits in combining massage
with some forms of exercise.
The immune system
The human immune system is a network of active and passive
defenses against substances and cells that would harm the body. It
includes innate immunity from barriers like the skin, body
temperature, pH (acidity) of the stomach, the inflammatory response
and the action of phagocytic cells. It also includes acquired
immunity, which is usually based on recognition and response to an
antigen. This generally involves white blood cells called
lymphocytes. There are two kinds: T cells (from the thymus) and B
cells (from the bone marrow). Acquired immunity may be humoral,
meaning it involves substances like antibodies and cytokines that
are dissolved or suspended in the blood, or it may be cell-mediated,
involving the cytotoxic activity of specialized cells.
Because the effects of exercise on the immune system do not
involve antigens, such immune activity is fundamentally different
from what you might read about in a text on immunology.
The Acute Phase Response
PARTS of the immune system are
depressed following a workout. However, this is not to say that the
body is defenseless. There is a "rapid deployment" system called the
acute phase response that kicks in after trauma, and exercise is
generally interpreted by the body as trauma. Exercise subjects the
body to oxidative stress, and that generates reactive oxygen species
and other free radicals that act as alarm molecules. Also, the body
can sense potentially dangerous changes in osmolarity (e.g.
swelling), hyperthermia (heat), hypoxia (oxygen starvation), pH
(acidity) of the blood, ionic contents of cells, and a variety of
other conditions.
Once initiated, the response is in the form of a cascade. Local
to the injury there is acute inflammation and blood clotting.
Systemically there is fever, leukocytosis (increased white blood
cells), increased levels of hormones like cortisol, and in
particular a major increase in synthesis of proteins called acute
phase proteins (ACPs). Let's look at this process in more detail.
Initialization
During exercise, free radicals known as reactive oxygen
intermediates (ROIs) and reactive nitrogen intermediates (RNIs) are
formed. Additionally other reactive intermediates such as carbonyls
may be produced. All these free radicals can signal and in some
cases activate cells of the immune system.
When intense exercise causes damage to cells, the contents of the
breached cell enters the surrounding lymph. This also has the effect
of signalling that there has been damage.
Monocytes are white blood cells with a single nucleus that are
formed in the bone marrow. When they arrive in the tissues of the
body they may differentiate (mature) into macrophages, and lose some
of their motility (ability to move independently). Muscle tissue
contains a number of macrophages, and these are the first immune
cells to react to exercise trauma. When a macrophage is activated,
it undergoes a "respiratory burst" of oxidation, which produces even
more ROIs, thus extending the signal to surrounding cells.
Macrophages also secrete signal molecules like IL-8 which act as
chemokines to attract other immune cells, in a process called
chemotaxis.
Prostaglandins are secreted by macrophages as well. These, plus
some metabolic byproducts of exercise like lactic acid, physical
changes involved in pumping the muscle, and the effects of the first
immune phenomena just described, combine to initiate inflammation in
the effected muscles.
Neutrophils in the blood sense the alarm molecules and
chemokines, and race to the defense of the injured tissue. The
inflamed blood vessels are more permeable, and in a process called
extravasation the neutrophils escape from the bloodstream, enter the
muscles, and home in on the damage. They in turn are activated,
undergo a respiratory burst, and begin secreting cytokines.
Cytokines
The first cytokines to be released as a result of exercise are
the "pro-inflammatory" substances interferon-gamma (IFN-gamma),
tumor necrosis factor (TNF) and interleukin-1 (IL-1). Also the
chemokine IL-8 is released. IFN-gamma, TNF and IL-1 have a number of
different effects on the body. They travel through the bloodstream
and stimulate the liver to synthesize acute phase proteins like
C-reactive protein, serum amyloid A and fibrinogen. They influence
complement, which is yet another factor in the immune system, and
kinins, which can produce vasodilation, pain, and may make you lose
your lunch in the squat rack. They cause body temperature to
increase. Most important for this article, all three act on T
lymphocytes to cause them to secrete interleukin-6.
IFN-gamma, TNF and IL-1 all have the reputation of being
catabolic cytokines which will reduce muscle mass. For example, IL-1
activates the enzyme "branched-chain alpha-keto acid dehydrogenase"
to oxidize amino acids in the muscles (Cannon1991). However as we'll
see below, at least part of the wasting effect may be mediated by
IL-6, so that if the effect of IL-6 is blocked some of the
catabolism is stopped.
T cell activation
A second part of the cytokine cascade derives from activated
lymphocytes. As we've mentioned, under normal exercise conditions,
immune cells are not activated by antigens. There are alternative
methods by which they can be activated. For example, lymphocyte
proliferation can be artificially stimulated with a chemical that
increases the level of glutathione, an antioxidant (Berridge1997).
Also it is known that reactive oxygen intermediates like hydrogen
peroxide ( H2O2 ) can activate the nuclear transcription factor
NF-kappaB. So there is good reason to expect that the
reduction/oxidation changes resulting from exercise may result in T
cell activation.
Also it is known that certain cytokines can activate lymphocytes.
For example, IL-1 was originally called "Lymphocyte Activating
Factor."
B lymphocytes are involved in antigen-based antibody formation,
so although they also secrete some cytokines we won't consider them
further. T cells differentiate under the influence of cytokines into
cytotoxic T lymphocytes and T helper (Th) cells. We need only
consider the latter. Th cells in turn differentiate into type 1 and
type 2 T helper cells (Th1 and Th2). It is the Th2 cells that
produce the bulk of the interleukin-6, although macrophages also
produce it, and even muscle cells seem to produce some under stress.
We'll cover IL-6 in detail below.
Termination of the acute phase
Cytokines and acute phase proteins have a brief half- life in the
body, so even without anti-inflammatory signalling this phase would
inevitably end. However a number of substances produced by the body
have the effect of bringing it to a conclusion more quickly.
As we all know, cortisol is secreted as a result of exercise. A
product of the adrenal glands, cortisol is a member of a class of
compounds called glucocorticoids. We normally think of
glucocorticoids as being catabolic, but they also has the effect of
inhibiting the synthesis of all acute phase cytokines. Since many of
those cytokines are catabolic, this is actually an anti-catabolic
action of cortisol. To put this in another way: if you are
successful in limiting cortisol production after a workout, you
might find an increased level of cytokines, and thus no net
prevention of muscle loss!
Cytokines sometimes have soluble receptors. One way these are
produced is from membrane receptors that are cleaved and "shed" from
cells. The receptors then circulate in the blood. Soluble receptors
for IL-1, IL-4 and TNF have the effect of binding to and thus
deactivating their cytokines. You might say they "mop up" the
cytokine. The production of these soluble receptors is a second way
in which the body limits the acute phase. On the other hand, soluble
receptors for IL-6 have the opposite effect: they can cause IL-6
metabolic effects on cells with incomplete receptors that normally
wouldn't be effected.
A protein called IL-1 receptor antagonist (IL-1Ra) binds to IL-1
receptors, blocking the effect of IL-1. IL-1Ra is secreted from
cells upon stimulation by TNF, and its production is enhanced by
IL-10 and IL-4. As levels of these last interleukins rise, IL-1
declines.
IL-10, IL-4 and IL-13 are anti-inflammatory cytokines. They
inhibit the production of inflammatory cytokines and also reduce
induction of cyclooxygenase-2 (COX2), an enzyme involved in
inflammation which is the target of drugs like aspirin. Whereas IL-1
and TNF are produced early in the acute phase response, the
anti-inflammatory cytokines come from activated T cells, so they are
a way in which the body gracefully concludes the acute phase.
IL-6
INTERLEUKIN-6 stands out among the
interleukins in several ways. It is the main signal of tissue damage
in the body (Sehgal1995). Although IL-1 and interferon initiate the
synthesis of some acute phase proteins by the liver, IL-6 stimulates
the liver to produce a larger and more complete set of these
proteins (Hilton1992, Baumann1987). IL-6 is thought by many
investigators to be the main factor in cachexia -- the wasting
syndrome that accompanies AIDS, cancer, and some autoimmune
diseases. Yet IL-6 is also a growth factor, intimately involved in
the production of new cells, including new muscle cells.
A better understanding of the pleiotropic roles of interleukin-6
should provide insight into methods of improving physical
development through training, nutrition and supplementation.
The IL-6 family of cytokines
IL-6 belongs to a family of physically similar or "homologous"
cytokines, including Leukemia Inhibitory Factor (LIF), Ciliary
Neurotropic Factor (CNTF), Granulocyte-Colony Stimulating Factor
(G-CSF), IL-11, Oncostatin M (OSM), and Cardiotropin-1 (CT-1). IL- 6
type cytokines feature four anti-parallel helices, arranged as shown
in Figure 1.
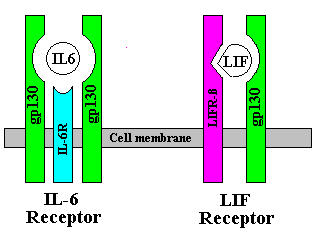
Receptors for IL-6 family cytokines are mulitimeric, having a
specific component for binding with the cytokine, plus a
transmembrane transducer protein called gp130 for delivering the
signal to the nucleus of the cell. For example, the LIF receptor is
composed of a gp130 molecule plus a specific component called LIFR-beta.
IL-6 receptor is a trimer, with two gp130 molecules, plus a specific
component called IL-6R-alpha. When IL-6 first contacts the cell, it
binds with IL-6R-alpha. Then the gp130 molecules dimerize and bind
with it to form the ligand-receptor complex.
While all this may appear a bit technical, study of the receptors
and how they are bound tells us much about the actions of these
cytokines. By means of this knowledge we can often block their
effects.
Signal transduction
Once the IL-6 receptor complex is assembled and bound, chemicals
within the cells called Janus kinases (JAK) phosphorylate the amino
acid tyrosine on the gp130 molecules. We have an effective tyrosine
kinase inhibitor (genistein) that can block this process. We'll
return to genistein in the section on IL-6 blockers, below.
The phosphotyrosines link up with a substance previously termed
"acute phase response factor" (APRF), but which is now called STAT3
(for "Signal Transducer and Activator of Transcription"). STAT1 and
STAT3 become phosphorylated and dimerize. Then these dimers travel
to the nucleus of the cell. Meanwhile the IL-6/IL-6R-alpha
combination is taken into the cell ("endocytosed") and is broken
down and destroyed. The gp130 units are recycled.
The STAT dimers bind with IL-6 response elements which then
activate gene transcription factors.
NF-IL-6 (Nuclear Factor IL-6) is a member of the C/EBP
(CAAT/Enhancer Binding Protein) family of transcription factors. It
is almost undetectable in normal circumstances, but when cells are
stimulated with IL-6 it is produced abundantly. C/EBP regulates fat
tissue. It increases differentiation from pre-adipocytes to
adipocytes, activates the glucose transporter GLUT4, etc. In short,
it makes you fat. When adipose tissue is treated with TNF -- which
reduces fat -- C/EBP is reduced, but NF-IL6 increases. It seems that
the ratio of C/EBP to NF-IL-6 is a determinant of fatness. Both IL-6
and LIF are known to drastically reduce fat, so the activation of
NF-IL-6 may be one of the mechanisms of that fat reduction.
STAT3 can also bind to the IL-6 response element of the junB gene
(JRE-IL6).
Apart from the JAK/STAT pathway, there is a second pathway from
the IL-6 receptor to the nucleus. It involves a protein called ras,
and Mitogen Activated Protein Kinase (MAPK).
As a result of the alternate pathways, a variety of transcription
factors can be activated, including AP-1 (Activator Protein-1) and
NF-kappaB (Nuclear Factor kappa B).
NF-kappa B
NF-kappaB deserves special mention. The name derives from its
discovery in B cells expressing kappa immunoglobulin. Subsequently
it was found that NF-kappaB exists in nearly all mammalian cells. It
regulates inflammation, immune reactions and acute phase response,
and it is generally bad news for athletes. Elderly people and people
with AIDS or chronic inflammation may have NF-kappaB almost
permanently activated, which accounts for some of the tissue loss
and poor health in those groups. On the other hand, NF-kappaB
regulated genes encode hematopoietic growth factors, which can be
useful to athletes.

Nuclear Factor kappa B
NF-kappaB is activity is low is a normal cell, due to an
inhibitor named I-kappa-B. IL-1 and TNF act to degrade I-kappa- B,
and by this means NF-kappaB is activated. As a result of
transcription regulated by NF-kappaB, many cytokines -- including
Il-6 -- are expressed. This is one way that IL-1 and TNF induce the
secretion of Il-6. NF-kappaB can also be activated by reactive
oxygen intermediates, and by IL-6 as described in the previous
section.
There are several effective methods of inhibiting NF-kappaB, some
of which will be described below.
Effects of
IL-6
MYOGENESIS -- the creation of new
muscle tissue -- occurs when muscle satellite cells (also called
sarcoplasts) or myoblasts (also called sarcoblasts) are activated.
Often the terms "myoblast" and "satellite cells" are used
interchangeably. Once activated, these cells proliferate, and then
differentiate, and finally fuse with other cells to form myotubes or
to join existing muscle fibers. The signal for these cells to
proliferate is Hepatocyte Growth Factor (HGF). HGF is induced by
heparin, which is liberated from the basal lamina of muscles when
they are damaged. It is also induced by interferon-gamma, and is
very potently induced by prostaglandin E2. All of these substances
appear as a result of trauma to the muscle. In addition to
activating the myoblasts, HGF increases their motility, so that they
can migrate to the site of damaged muscle.
This same trauma results in the expression of IL-6, LIF, and
Fibroblast Growth Factor (FGF). These three act as growth factors
(yes, in this case IL-6 is a growth factor), increasing the
proliferation of myoblasts. See Figure 3: response of IL-6 and LIF
to muscle injury (source: Kurek1996). LIF is a stronger inducer of
proliferation than IL-6, and whereas the effect of IL-6 is
short-lived, a brief exposure to LIF will result in proliferation
over an extended period. Injections of LIF have been suggested as a
therapy for muscle trauma and disease (Kurek1996).
When cells divide, the telomeres at the end of their chromosomes
shorten. Since the telomeres become shorter with each division, this
sets a limit (the "Hayflick limit") on the number of times that a
cell and its descendent cells can divide. This is particularly
important in germ cells like myoblasts. An enzyme named telomerase
can prevent the telomeres from shortening. Certain cytokines,
including IL-6, can induce telomerase, hence increasing the number
of times a cell can divide (Engelhardt1997). This appears to be a
unique contribution made by IL-6 to the muscle regeneration system.
IL-6 also has a similar effect in hemopoietic tissue.
Effect on Fat
Experiments on mice that were reported in 1989 and 1990 showed
LIF inhibits the action of lipoprotein lipase (LPL), which is
instrumental in uptake of fatty acids by adipose tissue
(Hilton1992). A "dramatic and rapid loss of virtually all
subcutaneous and abdominal fat" was reported. More recently, it has
been shown that while administration of recombinant IL-6 to mice
reduces LPL, it has almost no effect on fat reduction in mice
(Fujita1996).
We've previously mentioned that IL-6 activates a regulator of fat
tissue called NF-IL-6. NF-IL-6 is actually a repressed transcription
factor which is normally inhibited. Signalling from IL-6 through the
MAP kinase pathway overcomes the inhibition (Akira1995). In this
way, while IL-6 may not be as successful at blocking fat uptake as
LIF, it may decrease body fat by slowing the maturation of
adipocytes.
Effect on Muscle
There are three main proteolytic pathways in skeletal muscle:
cathepsins functioning in the lysosome, calpain proteases in the
cell's cytosol, and the ATP-ubiquitin (Ub) pathway. IL-6 acts to
destroy muscle through the cathepsin and ATP-Ub pathways. Fujita et
al. showed that mice inoculated with a cancer (adenocarcinoma)
developed high levels of IL-6 after 11 days: while untreated control
mice had a level of 7.9 pg/ml of IL- 6, inoculated mice had an
average of 1,142 pg/ml (Fujita1996). These inoculated mice had
cathepsin B levels 236% higher, and cathepsin B levels 826% higher
than controls. Tsujinaka et al. showed that in transgenic mice
carrying DNA for human IL-6, treated mice had cathepsin B levels 20
times higher than controls (Tsujinaka1996).
IL-6 shortens the half-life of proteins in the myotubes that make
up muscle fibers. It has been demonstrated that mRNA levels of
proteosomes, which are involved with the ATP-Ub pathway, are
increased by IL-6 (Ebisui1995, Tsujinaka1996). Strangely, TNF, which
is often named as the main culprit in cachexia (wasting syndrome),
has not been shown to have this effect. In fact, several studies
have failed to show a direct effect by TNF on muscle proteolysis
(reviewed in Fujita1996). Therefore it seems that the proteolytic
action of TNF may actually be mediated through IL-6. In other words,
without IL-6, TNF would not destroy muscle (although it would reduce
fat). Therefore, IL-6 appears to be the primary agent in muscle
wasting.
IL-6 is a catabolic agent in many disease states
(Papanicolaou1998). It is present in rheumatism and other autoimmune
type diseases, and is responsible for joint deterioration and muscle
loss. DeBenedetti et al. found that transgenic mice with human IL-6
had stunted growth, attaining only about half the size of normal
mice (Figure2). They also showed that in humans as well as mice,
there is a negative correlation between IL-6 and IGF-1. In other
words, the more IL-6 in the body, the less IGF-1. This relationship
was unique to IL- 6: TNF and IL-1 were not correlated with IGF-1
(DeBenedetti1997).
In summary, the effects of IL-6 are mostly harmful for athletes.
It plays beneficial roles in resisting infection, stimulating the
acute phase response in case of trauma, and in hematopoiesis and
production of stem cells. However, excessive IL-6 resulting from
exercise or chronic inflammation will destroy muscle tissue and
reduce IGF-1.
Manipulation
of IL-6
Now that we know the actions and effects of interleukin-6, let's
consider ways to manipulate IL-6 secretion.
In the unlikely event that we'd want more IL-6, the method
is obvious: just exercise. Any exercise that causes trauma to the
muscles would suffice. If we want to start an acute phase response
without the temporary immune suppression caused by exercise, there
are herbs like Echinaceae and Rudbeckia speciosa
that contain polysaccharides the body mistakes for bacteria, so that
they can initiate an immune response (Bukovsky1995).
For inhibition of IL-6 and its effects there are many options.
We'll first cover nutrition and supplements, and then drugs. Not all
the options mentioned will be suitable for athletes; the goal here
is to compile a comprehensive list from which people can choose
according to their needs.
Nutrition and Supplements
Caloric restriction is perhaps the simplest method of
reducing IL-6 (Volk1994). This is a technique employed by life-
extensionists. It might have some application to dieting
athletes, providing they don't use weight-loss drugs.
Oils and fatty acids.
oEicosapentaenoic acid (EPA) supplementation significantly
reduced IL-6 ecretion from mononuclear cells in patients with cancer
(Wigmore1997).
oSupplementation with docosahexaenoic acid (DHA) and EPA
reduced production of IL-1, IL-6, TNF and IL-2 by mononuclear cells
in normal individuals and in patients with Rheumatoid Arthritis and
with Multiple Sclerosis (Calder1997).
o The fatty acids gamma linolenic acid (GLA), EPA and DHA
reduce serum IL-1, IL-2, IL-4, IL-6, TNF alpha and IFN-gamma in
cancer patients. Three months after cessation of fatty acid
supplementation cytokine values returned to normal (Purasiri1994).
o In human endothelial cells, DHA decreased secretion of
IL- 6, IL-1, IL-4, IL- 8 and TNF (DeCaterina1994).
o Blackcurrant seed oil rich in GLA reduced production of
IL- 1 beta, TNF alpha IL-6 and PGE2 (Watson1993).
Sources of GLA: evening primrose oil, borage seed oil,
blackcurrant seed oil.
Sources of EPA and DHA: fish oils (e.g. cod liver oil, salmon
oil, etc.).
Lactoferrin (found in milk) reduces IL-6 (Mattsby1996).
Estrogen and androgens reduce IL-6 and block NF-kappaB.
Therefore, foods like soy which are estrogenic and
supplements like androstenedione would be expected to
have a similar effect.
Since one of the signal pathways from the IL-6 receptor
depends on tyrosine kinase, genistein, which is an
effective tyrosine kinase inhibitor, should block it. Genistein
is a component of soy, and can be purchased in purified form.
Zinc induces Heat Shock Protein HSP-70 and reduces
cytokines and apoptosis (Klosterhalfen1997).
Antioxidants. Since antioxidants provide some of the initial
signals in the acute phase response, and since NF-kappaB can be
directly activated by reactive oxygen intermediates,
antioxidants can prevent secretion of IL-6 and the effects of
NF-kappaB transcription.
o Vitamin E supplementation (400 units twice per day)
almost completely eliminated increased secretion of Il-6 in athletes
following three 15 minute sets of downhill running (Cannon1991).
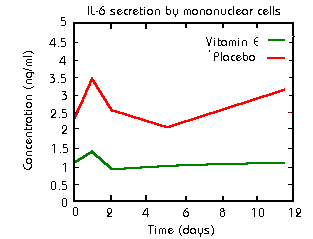
o L-ascorbic acid inhibits secretion of IL-1 and IL-6
(Tebbe1997).
o Black tea extract lowers concentrations of IL-6
(Amarakoon1995).
o Melatonin reduces oxidative stress, improves immune
function. etc. (Reiter1997).
o Since expression of IL-6 mRNA is dependent on NF-kappaB binding
to the IL-6 gene, supplementation with the antioxidant
N-acetyl-L-cysteine (NAC) can block the process (Shibanuma1994).
o A number of experimental antioxidants have been employed
in studies of NF-kappaB inhibition. They include glutathione, NADPH,
pyrrolidine dithiocarbamate (PDTC), butylated hydroxyanisole (BHA),
and various forms of superoxide dismutase.
Since IL-1, IFN-gamma, and TNF induce IL-6 production, any
substances that inhibit them will usually have the effect of
inhibiting Il-6.
NF-kappaB can be inhibited by nitric oxide (NO). One
substance that induces NO is the amino acid arginine.
Aspirin and salicylate inhibit NF-kappaB. Also salicylate
inhibits protein kinase activity, and so would prevent
signalling by IL-6 via tyrosine kinase (Beauparlant1996). Since
methyl salicylate is a common ingredient of ointments for sore
muscles, this raises the possibility that a topical application
could be effective against IL-6.
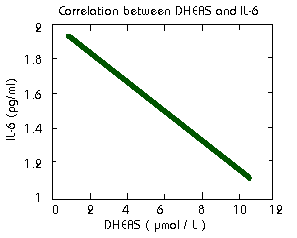
There is a negative correlation between dehydroepiandrosterone
sulfate (DHEAS) and IL-6 in the blood (Straub1998). Therefore
supplementation with DHEA will reduce IL-6.
Drugs for IL-6 reduction
Since IL-6 is a factor in many diseases, a number of drugs and
pharmaceutical techniques have been investigated for lowering IL- 6
levels. Some of the substances mentioned below are experimental or
unapproved.
Glucocorticoids like dexamethasone block transcription
factors NF-kappaB and AP-1 (Brattsand1996). Unfortunately they
are also catabolic to muscle, and so are of little use to the
athlete, except in case of injury.
RU486 (mifepristone) can block NF-kappaB induced by TNF,
although not as well as glucocorticoids (Beauparlant1996). It
has the advantage that it also blocks glucocorticoid receptors,
but unfortunately the receptors soon upregulate.
The immunosuppressant drugs FK506 and cyclosporin A
will suppress T cells, but this would be an insane way to
inhibit IL-6, due to the side effects.
Anti-inflammatory cytokines like IL-10, IL-4, IL-13, and
Transforming Growth Factor beta (TGF-beta) will inhibit
synthesis of IL-6 and other inflammatory cytokines. IL-10 also
enhances synthesis of IL-1 receptor antagonist, downregulates
TNF receptors and inhibits T cell proliferation (Koj1998,
Xing1997, Dokter1996).
Soluble cytokine receptors and receptor antagonists are
effective against IL-1 and TNF, which induce IL-6.
Unfortunately, the IL-6 soluble receptor only increases the
effect of IL-6. Enbrel, a soluble receptor for TNF made
by Immunex, will be on the market soon for treatment of
rheumatoid arthritis and similar inflammatory conditions.

Rolipram, an antidepressant sold in Europe by Schering,
is also very effective at inhibiting TNF, and so has an indirect
effect on IL-6.
Tenidap, a new anti-rheumatic drug, showed a great deal
of promise against cytokines, but the FDA decided not to approve
it because of problems with proteinuria (protein in the urine).
This side effect may make it unsuitable for athletes. It is
available from Europe (Breedveld1994, Bondeson1996).
Polymyxin B administration results in a prompt reduction
in interleukin-6 levels in burn patients (Cone1997).
For women, medroxyprogesterone acetate has reduced
IL-6 in breast cancer patients. Reduction was correlated with
plasma levels of MPA, not dosage (Yamashita1996).
Use of a monoclonal antibody against CD-54 (ICAM-1)
reduced IL-6 in rheumatoid arthritis (RA) patients
(Schulze1996).
Antibodies against TNF have been used to reduce IL-6
(Fekade1996).
Indomethacin reduces Il-6 by inhibiting prostaglandin E2
(Hinson1996).
Tyloxapol, a potent anti-oxidant used in the treatment of
cystic fibrosis and chronic bronchitis, inhibits NF-kappaB and
IL-6 (Ghio1996).
The anti-rheumatic drug minocycline decreases serum
levels of IL-6 (Kloppenburg1996).
The anti-rheumatic drug tepoxalin inhibits the
production of IL-2, IL-6 and TNF alpha and inhibits activation
of NF- kappaB (Ritchie1995).
Pentoxifylline is a methylxanthine derivative that acts
as a phosphodiesterase inhibitor and is prescribed to improve
capillary flow. It inhibits TNF and IL-6 and counteracts the
respiratory burst of phagocytes that produces free radicals
(Lundblad1995), Koj1998, Mandell1995).
Torbafylline, a xanthine derivative that suppresses TNF,
has been used with some experimental success in the treatment of
cachexia (Sinha1995).

The sex hormones estrogen and testosterone block IL-6
(Bellido1995, Vaananen1996, Stein1995). In fact, it seems that
nearly any steroid inhibits IL-6: estrogen, testosterone,
DHEA, glucocorticoids, and probably most androgenic/anabolic
drugs.
Angiotensin Converting Enzyme (ACE) inhibitors decrease
the levels of angiotensin II or limits its action, thereby
interfering with the permissive effect of Angiotensin on IL-6
(Klahr1998).
Antibodies that destroy IL-6 receptors are effective at
preventing muscle proteolysis caused by IL-6 (Fujita1996).
Conclusion
EXERCISE activates the immune
system, which then cycles through an abbreviated version of the
acute phase response. Damage to muscles results in IL-6 secretion,
which signals the body to produce acute phase proteins. Depending on
the amount of muscle damage, the acute phase response will terminate
sooner or later, by the action of cortisol and anti-inflammatory
cytokines.
IL-6 is the main mediator of muscle wasting. It may have some
beneficial actions at the onset of the acute phase response, but
chronically high IL-6 levels must be avoided for good health and
optimum muscular development. We have a number of ways to accomplish
that, from the simple use of antioxidants to specially designed
antibodies. Through the use of these agents in coordination with
training activity, we can effectively reduce the unnecessary muscle
breakdown that normally follows intense exercise.
References
1. Agnello D, Meazza C, et al. 1998 Sep. Leptin causes
body weight loss in the absence of in vivo activities typical of
cytokines of the IL-6 family. Am J Physiol. 275(3 Pt 2):R913-9.
2. Akira S, Yoshida K, Tanaka T, Taga T, Kishimoto T.
1995. Targeted disruption of the IL-6 related Genes: gp130 and
NF-IL-6. Immunol Rev. 148:221-53.
3. Amarakoon AM, Tappia PS, Grimble RF. 1995. Endotoxin
induced production of interleukin-6 is enhanced by vitamin E
deficiency and reduced by black tea extract. Inflamm Res.
44:301-305.
4. Ambrus JL Jr, Pippin J, et al. 1993 Jul 1.
Identification of a cDNA for a human high-molecular-weight B-cell
growth factor. Proc Natl Acad Sci U S A. 90(13):6330-4.
5. Austin L, Burgess AW. 1991 Feb. Stimulation of myoblast
proliferation in culture by leukaemia inhibitory factor and other
cytokines. J Neurol Sci. 101(2):193-7.
6. Barnoy S, Glaser T, Kosower NS. 1998 Mar 12. The
calpain-calpastatin system and protein degradation in fusing
myoblasts. Biochim.Biophys.Acta 1402(1):52-60.
7. Baumann H, Onorato V, Gauldie J, Jahreis GP. 1987 Jul 15.
Distinct sets of acute phase plasma proteins are stimulated by separate
human hepatocyte-stimulating factors and monokines in rat hepatoma
cells. J.Biol.Chem. 262(20):9756-68.
8. Baumann H, Morella KK, et al. 1996 Aug 6. The full-length
leptin receptor has signaling capabilities of interleukin 6-type
cytokine receptors. Proc Natl Acad Sci U S A. 93(16):8374-8.
9. Beauparlant P, Hiscott J. 1996. Biological and biochemical
inhibitors of the NF-kappaB/Rel proteins and cytokine synthesis.
Cytokine Growth Factor Rev. 7(2):175-190.
10. Bellido T, Jilka RL, et al. 1995 Jun. Regulation of
interleukin-6, osteoclastogenesis, and bone mass by androgens. The role
of the androgen receptor. J.Clin.Invest. 95(6):2886-95.
11. Berkowitz DE, Brown D, et al. 1998 Jun. Endotoxin-induced
alteration in the expression of leptin and beta3-adrenergic receptor in
adipose tissue. Am J Physiol. 274(6):E992-E997.
12. Berridge MJ. 1997. Lymphocyte activation in health and
disease. Crit Rev Immunol. 17:155-178.
13. Bischoff R. 1997 Apr. Chemotaxis of skeletal muscle
satellite cells. Dev.Dyn. 208(4):505-15.
14. Blackwell TS, Christman JW. 1997 Jul. The Role of Nuclear
Factor-kappa B in Cytokine Gene Regulation. Am J Respir Cell Mol Biol.
17(1):3-9.
15. Bondeson J. 1996. Effects of tenidap on intracellular
signal transduction and the induction of proinflammatory cytokines: a
review. Gen Pharmac. 27:943-956.
16. Boyum A, Wiik P, et al. 1996 Feb. The effect of strenuous
exercise, calorie deficiency and sleep deprivation on white blood cells,
plasma immunoglobulins and cytokines. Scand J Immunol. 43(2):228-35.
17. Brakenhoff JPJ, DeHon FD, Aarden LA. 1995 Jul 21.
Development of human IL-6 receptor antagonists. Ann N Y Acad Sci.
762:129-135.
18. Brattsand R, Linden M. 1996. Cytokine modulation by
glucocorticoids: mechanisms and actions in cellular studies. Aliment
Pharmacol Ther. 10(Suppl 2):81-90.
19. Breedveld F. 1994. Tenidap: a novel cytokine-modulating
antirheumatic drug for the treatment of rheumatoid arthritis. Scand J
Rheumatol Suppl. 100:31-44.
20. Bruunsgaard H, Galbo H, et al. 1997 Mar 15.
Exercise-induced increase in serum interleukin-6 in humans is related to
muscle damage. J Physiol (Lond). 499 ( Pt 3):833-41.
21. Bukovsky M, Vaverkova S, Kost'alova D. 1995 Mar-Apr.
Immunomodulating activity of Echinacea gloriosa L., Echinacea
angustifolia DC. and Rudbeckia speciosa Wenderoth ethanol-water
extracts. Pol J Pharmacol. 47(2):175-7.
22. Calder, PC. 1997. n-3 polyunsaturated fatty acids and
cytokine production in health and disease. Ann Nutr Metab 41: 203-234
23. Cannon JG, Meydani SM, et al. 1991. Acute phase response
in exercise. II. Associations between vitamin E, cytokines, and muscle
proteolysis. Am J Physiol. 260:R1235-R1240.
24. Cannon JG, Fiatarone MA, Fielding RA, Evans WJ. 1994 Jun.
Aging and stress-induced changes in complement activation and neutrophil
mobilization. J.Appl.Physiol. 76(6):2616-20.
25. Center DM, Kornfeld H, Cruikshank WW. 1997 Nov.
Interleukin-16. Int J Biochem Cell Biol. 29(11):1231-4.
26. Cone JB, Wallace BH, Lubansky HJ, Caldwell FT. 1997.
Manipulation of the inflammatory response to burn injury. J Trauma
43:41-45.
27. DeBenedetti F, Alonzi T, et al. 1997 Feb. Interleukin 6
Causes Growth Impairment in Transgenic Mice through a Decrease in
Insulin-like Growth Factor-I: A Model for Stunted Growth in Children
with Chronic Inflammation. J. Clin. Invest. 99(4):643-650.
28. De Caterina R, Cybulsky MI, et al. 1994. The omega-3 fatty
acid docosahexaenoate reduces cytokine- induced expression of
proatherogenic and proinflammatory proteins in human endothelial cells.
Arterioscler Thromb. 14:1829-1836.
29. Dendorfer U, Oettgen P, Libermann TA. 1994. Multiple
regulatory elements in the interleukin-6 gene mediate induction by
prostaglandins, cyclic AMP, and lipopolysaccharide. Mol Cell Biol.
14:4443-4454.
30. Dinarello CA, Novick D, Puren AJ, Fantuzzi G, Shapiro L, et
al. 1998 Jun. Overview of interleukin-18: more than an
interferon-gamma inducing factor. J Leukoc Biol. 63(6):658-64.
31. Dokter WA, Koopmans SB, Vellenga E. 1996. Effects of IL-10
and IL-4 on LPS-induced transcription factors (AP-1, NF-IL6 and NF-
kappa B) which are involved in IL-6 regulation. Leukemia. 10:1308- 1316.
32. Ebisui C, Tsujinaka T, et al. 1995. Interleukin-6 induces
proteolysis by activating intracellular proteases (cathepsins B and L,
proteasome) in C2C12 myotubes. Clin Sci (Colch). 89(4):431-9.
33. Engelhardt M, Kumar R, et al. 1997 Jul 1. Telomerase
regulation, cell cycle, and telomere stability in primitive
hematopoietic cells. Blood. 90(1):182-93.
34. Fekade DK, Knox K, et al. 1996. Prevention of
Jarisch-Herxheimer reactions by treatment with antibodies against tumor
necrosis factor alpha. N Engl J Med. 335:311-315.
35. Fujita J, Tsujinaka T, et al. 1996. Anti-interleukin-6
receptor antibody prevents muscle atrophy in colony-26
adenocarcinoma-bearing mice with modulation of lysosomal and
ATP-ubiquitin-dependent proteolytic pathways. Int J Cancer. 68:637-643.
36. Ghio AJ, Marshall BC, et al. 1996. Tyloxapol inhibits
NF-kappa B and cytokine release, scavenges HOCI, and reduces viscosity
of cystic fibrosis sputum. Am J Respir Crit Care Med. 154:783-788.
37. Heinrich PC, Horn F, Graeve L, Dittrich E, Kerr I,
Müller-Newen G, Grötzinger J, Wollmer A. 1998. Interleukin-6 and
related cytokines: effect on the acute phase reaction. Z Ernährungswiss.
37(Suppl 1):43-49.
38. Hibi M, Nakajima K, Hirano T. 1996. IL-6 cytokine family
and signal transduction: a model of the cytokine system. J Mol Med.
74:1-12.
39. Hilton DJ. 1992 Feb. LIF: lots of interesting functions.
TIBS. 17:72-76.
40. Hinson RM, Williams JA, Shacter E. 1996. Elevated
interleukin 6 is induced by prostaglandin E2 in a murine model of
inflammation: possible role of cyclooxygenase-2. Proc Natl Acad Sci USA.
93:4885-4890.
41. Hirano T, Nakajima K, Hibi M. 1997. Signaling mechanisms
through gp130 : a model of the cytokine system. Cytokine Growth Factor
Rev. 8:241-252.
42. Hirano T. 1998. Interleukin 6 and its receptor: Ten years
later. Internat Rev Immunol. 16:249-284.
43. Husebekk A, Stenstad T, et al. 1996. Experimental
AA-amyloidosis in mice is inhibited by treatment with the anti-rheumatic
drug tenidap. Scand J Immunol. 43:551-555.
44. Ironson G, Field T, et al. 1996 Feb. Massage therapy is
associated with enhancement of the immune system's cytotoxic capacity.
Int J Neurosci. 84(1-4):205-17.
45. Klahr S, Morrissey J. 1998 (Jan). Angiotensin II and gene
expression in the kidney. Am J Kidney Dis. 31(1):171-6.
46. Kloppenburg M, Dijkmans BA, et al. 1996. Inflammatory and
immunological parameters of disease activity in rheumatoid arthritis
patients treated with minocycline. Immunopharmacology. 31:163-169.
47. Klosterhalfen B, Hauptmann S, et al. 1997. Induction of
heat shock protein 70 by zinc-bis-(DL- hydrogenaspartate) reduces
cytokine liberation, apoptosis, and mortality rate in a rat model of
LD100 endotoxemia. Shock. 7:254-262.
48. Koj A. 1996. Initiation of acute phase response and
synthesis of cytokines. Biochim Biophys Acta. 1317:84-94.
49. Koj A. 1998. Termination of acute-phase response: role of
some cytokines and anti-inflammatory drugs. Gen Pharmac. 31(1):9-18.
50. Kurek JB, Nouri S, Kannourakis G, Murphy M, Austin L.
1996. Leukemia inhibitory factor and interleukin-6 are promoted by
diseased and regenerating skeletal muscle. Muscle Nerve. 19:1291-1301.
51. Lai CF, Ripperger J, Morella KK, Jurlander J, Hawley TS,
Carson WE, Kordula T, Caligiuri MA, Hawley RG, Fey GH, Baumann H.
1996 Jun 14. Receptors for interleukin (IL)-10 and IL-6-type cytokines
use similar signaling mechanisms for inducing transcription through IL-6
response elements. J.Biol.Chem. 271(24):13968-75.
52. Leon LR, White AA, Kluger MJ. 1998 Jul. Role of IL-6 and
TNF in thermoregulation and survival during sepsis in mice. Am J
Physiol. 275(1):R269-R277. (Full text - subscription required)
53. Lin SC, Yamate T, Taguchi Y, Borba VZ, Girasole G, O'Brien CA,
Bellido T, Abe E, Manolagas SC. 1997 Oct 15. Regulation of the gp80
and gp130 subunits of the IL-6 receptor by sex steroids in the murine
bone marrow. J Clin Invest. 100(8):1980-90.
54. Lundblad R, Ekstrom P, Giercksky KE. 1995. Pentoxifylline
improves survival and reduces tumor necrosis factor, interleukin-6, and
endothelin-1 in fulminant intra-abdominal sepsis in rats. Shock.
3:210-215.
55. Mandell GL. 1995. Cytokines, phagocytes, and
pentoxifylline. J Cardiovasc Pharmacol 25 Suppl 2: S20-S22.
56. Mattsby-Baltzer I, Roseanu A, Motas C, Elverfors J, Engberg I,
Hanson LA. 1996. Lactoferrin or a fragment thereof inhibits the
endotoxin-induced interleukin-6 response in human monocytic cells.
Pediatr Res. 40:257-262.
57. Mertsching E, Meyer V, Linares J, Lombard-Platet S, Ceredig R.
1998. Interleukin-7, a non-redundant potent cytokine whose
over-expression massively perturbs B-lymphopoiesis. Int Rev Immunol.
16(3-4):285-308.
58. Nieman DC. 1997 May. Immune response to heavy exertion. J
Appl Physiol. 82(5):1385-94.
59. Ounissi-Benkalha H, Pelletier JP, et al. 1996. In vitro
effects of 2 antirheumatic drugs on the synthesis and expression of
proinflammatory cytokines in synovial membranes from patients with
rheumatoid arthritis. J Rheumatol. 23:16-23.
60. Papanicolaou DA, Petrides JS, et al. 1996 Sep. Exercise
stimulates interleukin-6 secretion: inhibition by glucocorticoids and
correlation with catecholamines. Am J Physiol. 271(3 Pt 1):E601-5.
61. Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP.
1998 Jan 15. The pathophysiologic roles of interleukin-6 in human
disease. Ann.Intern.Med. 128(2):127-37.
62. Pedersen BK, Bruunsgaard H, et al. 1997 Mar.
Exercise-induced immunomodulation--possible roles of neuroendocrine and
metabolic factors. Int J Sports Med. 18(Suppl 1):S2-7.
63. Purasiri P, Murray A, Richardson S, Heys SD, Horrobin D,
Eremin O. 1994. Modulation of cytokine production in vivo by dietary
essential fatty acids in patients with colorectal cancer. Clin Sci
(Colch.) 87:711-717.
64. Quinn LS, Haugk KL, Grabstein KH. 1995 Aug.
Interleukin-15: a novel anabolic cytokine for skeletal muscle.
Endocrinology. 136(8):3669-72.
65. Reiter RJ, Carneiro RC, Oh CS. 1997 Aug. Melatonin in
relation to cellular antioxidative defense mechanisms. Horm Metab Res.
29(8):363-72.
66. Ritchie DM, Argentieri DC, et al. 1995.
Cytokine-modulating activity of tepoxalin, a new potential
antirheumatic. Int J Immunopharmacol. 17: 805-812.
67. Rohde T, MacLean DA, Richter EA, Kiens B, Pedersen BK.
1997. Prolonged submaximal eccentric exercise is associated with
increased levels of plasma IL-6. Am J Physiol. 273:E85-E91.
68. Rohde T, MacLean DA, Pedersen BK. 1998 Jun. Effect of
glutamine supplementation on changes in the immune system induced by
repeated exercise. Med.Sci.Sports Exerc. 30(6):856-62.
69. Roubenoff R. 1997 May 5. Inflammatory and Hormonal
Mediators of Cachexia. J Nutrition. 127(5):1014S-1016S.
70. Saba AA, Kaidi AA, et al. 1996. Effects of interleukin-6
and its neutralizing antibodies on peritoneal adhesion formation and
wound healing. Am Surg. 62:569-572.
71. Sancéau J, Kaisho T, Hirano T. 1995 Nov 17. Triggering of
the human interleukin-6 gene by interferon- and tumor necrosis factor-
in monocytic cells involves cooperation between Interferon Regulatory
Factor-1, NFB, and Sp1 transcription factors. 270(46): 27920-27931.
72. Schulze-Koops H, Lipsky PE, Kavanaugh AF, Davis LS. 1996.
Persistent reduction in IL-6 mRNA in peripheral blood mononuclear cells
of patients with rheumatoid arthritis after treatment with a monoclonal
antibody to CD54 (ICAM-1). Clin Exp Immunol. 106:190-196.
73. Sehgal PB, Wang L, Rayanade R, Pan H, Margulies L. 1995
Jul 21. Interleukin-6-type cytokines. Ann.N.Y.Acad.Sci. 762:1-14.
74. Sharp NC, Koutedakis Y. 1992 Jul. Sport and the
overtraining syndrome: immunological aspects. Br.Med.Bull. 48(3):518-33.
75. Shephard RJ, Rhind S, Shek PN. 1994 Oct. Exercise and
training: influences on cytotoxicity, interleukin-1, interleukin-2 and
receptor structures. Int J Sports Med. 15 Suppl 3:S154-66.
76. Shibanuma M, Kuroki T, Nose K. 1994 Oct 10. Inhibition by
N-acetyl-L-cysteine of interleukin-6 mRNA induction and activation of NF
kappa B by tumor necrosis factor alpha in a mouse fibroblastic cell
line, Balb/3T3. FEBS Lett. 353(1):62-6.
77. Sinha B, Semmler J, et al. 1995 Jan. Enhanced tumor
necrosis factor suppression and cyclic adenosine monophosphate
accumulation by combination of phosphodiesterase inhibitors and
prostanoids. Eur J Immunol. 25(1):147-53.
78. Speirs V, Adams EF, White MC. 1993. The anti-estrogen
tamoxifen blocks the stimulatory effects of interleukin-6 on 17 beta-
hydroxysteroid dehydrogenase activity in MCF-7 cells. J Steroid Biochem
Mol Biol. 46:605-611.
79. Spencer NFL, Poynter ME, Hennebold JD, Mu HH, Daynes RA.
1995 Jul 21. Does DHEAS restore immune competence in aged animals
through its capacity to function as a natural modulator of peroxisome
activities? Ann N Y Acad Sci. 762:200-216.
80. Spriggs MK. 1997 Sep. Interleukin-17 and its receptor. J
Clin Immunol. 17(5):366-9.
81. Stein B, Yang MX. 1995. Repression of the interleukin-6
promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta.
Mol Cell Biol. 15:4971-4979.
82. Straub RH, Konecna L, Hrach S, Rothe G, Kreutz M, et al.
1998. Serum dehydroepiandrosterone (DHEA) and DHEA sulfate are
negatively correlated with serum interleukin-6 (IL-6), and DHEA inhibits
IL-6 secretion from mononuclear cells in man in vitro: possible link
between endocrinosenescence and immunosenescence. J Clin Endocrinol
Metab. 83:2012-2017.
83. Tartaglia LA, Dembski M, et al. 1995. Identification and
expression cloning of a leptin receptor, OB-R. Cell. 83:1263-1271.
84. Tebbe B, Wu S, et al. 1997. L-ascorbic acid inhibits
UVA-induced lipid peroxidation and secretion of IL-1alpha and IL-6 in
cultured human keratinocytes in vitro. J Invest Dermatol. 108:302-306.
85. Tidball JG. 1995 Jul. Inflammatory cell response to acute
muscle injury. Med.Sci.Sports Exerc. 27(7):1022-32.
86. Tsujinaka T, Fujita J, et al. 1996 Jan 1. Interleukin 6
receptor antibody inhibits muscle atrophy and modulates proteolytic
systems in interleukin 6 transgenic mice. J Clin Invest. 97(1):244-9.
87. Ullum H, Haahr PM, et al. 1994 Jul. Bicycle exercise
enhances plasma IL-6 but does not change IL-1 alpha, IL-1 beta, IL-6, or
TNF-alpha pre-mRNA in BMNC. J Appl Physiol. 77(1):93-7.
88. Vaananen HK, Harkonen PL. 1996 May. Estrogen and bone
metabolism. Maturitas. 23 Suppl:S65-S69.
89. Volk MJ, Pugh TD, et al. 1994. Dietary restriction from
middle age attenuates age-associated lymphoma development and
interleukin 6 dysregulation in C57BL/6 mice. Cancer Res 54:3054-3061.
90. Wang P, Wu P, Siegel MI, Egan RW, Motasim Billah M. 1995.
Interleukin (IL)-10 Inhibits Nuclear Factor kappa B (NFKB) Activation in
Human Monocytes.J Biol Chem. 270(16):9558-9563.
91. Watson J, Byars ML, et al. 1993. Cytokine and
prostaglandin production by monocytes of volunteers and rheumatoid
arthritis patients treated with dietary supplements of blackcurrant seed
oil. Br J Rheumatol. 32:1055-1058.
92. Watson RR, Huls A, Araghinikuam M, Chung S. 1996 Oct.
Dehydroepiandrosterone and diseases of aging. Drugs Aging. 9(4):274-291.
93. Weinstock C, Konig D, et al. 1997 Mar. Effect of
exhaustive exercise stress on the cytokine response. Med Sci Sports
Exerc. 29(3):345-54.
94. Wigmore SJ, Fearon KC, Maingay JP, Ross JA. 1997.
Down-regulation of the acute-phase response in patients with pancreatic
cancer cachexia receiving oral eicosapentaenoic acid is mediated via
suppression of interleukin-6. Clin Sci (Colch.) 92:215-221.
95. Xing Z, Ohkawara Y, et al. 1997. Adenoviral
vector-mediated interleukin-10 expression in vivo: intramuscular gene
transfer inhibits cytokine responses in endotoxemia. Gene Ther
4:140-149.
96. Yamashita J, Hideshima T, Shirakusa T, Ogawa M. 1996.
Medroxyprogesterone acetate treatment reduces serum interleukin-6 levels
in patients with metastatic breast carcinoma. Cancer 78:2346-2352.
97. Yan SF, Tritto I, Pinsky D, Liao H, Huang J, Fuller G, Brett
J, May L, and Stern D. 1995 May 12. Induction of Interleukin 6
(IL-6) by Hypoxia in Vascular Cells. J Biol Chem. 270(19):11463-11471
|